31/08/2017
Scientists discover a new solvent-free method of cryopreserving red blood cells
SNAL project demonstrates improvements offered by utilising apatite nanoparticles in red blood cell cryopreservation
SNAL project demonstrates improvements offered by utilising apatite nanoparticles in red blood cell cryopreservation
Blood transfusion is an important and day-to-day based clinical practice. This procedure necessities a sufficient stock of red blood cells in blood blank. However, red blood cells used for transfusions have a short lifetime with standard storage conditions (only 42 days), and maintaining a constant supply of blood bank is always a persistent problem. Since the 70s, some solvents (like glycerol) have been used as cryoprotectant for red cells cryopreservation in hospital. But glycerol is toxic and should be removed by dialysis before the blood could be transfused to a patient. This dialysis step is slow (so, inconvenient for emergency), expensive and the red blood cells lifetime after thawing is only of a day. Natural cryoprotectant, like trehalose, could be used to cryopreserve red blood cells without needing any dialysis step. Nevertheless, the associated red blood cells survival rate is low which make this method unsuitable for clinical issues.
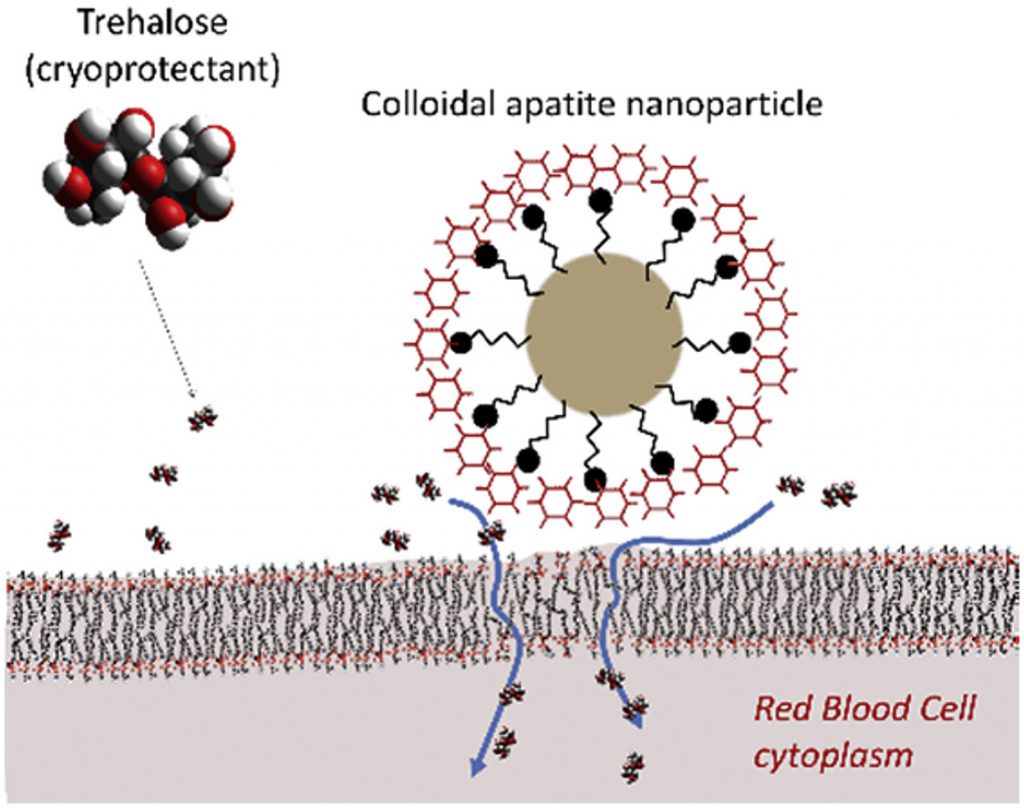
Based on these problems, the group of Christophe Drouet (CNRS- Toulouse) produced low-cost apatite nanoparticles (200nm in diameter) that are made with the same chemical components than bones and teeth, so perfectly biocompatible. Then in collaboration with Martin Stephanic (researcher in Biopharma, a company specialized in cryopreservation), they test to cryopreserve red blood cells in presence of these nanoparticles. The corresponding cells survival rate after thawing was still quite low, except if trehalose was also added to red blood cells. In that case, more than 90% of red blood cells could survive to thawing (without dialysis step) that would make this method suitable for clinical issues. These results indicated clearly that these nanoparticles were able to load the red cells with trehalose, but the associated mechanism was unclear.
To solve this question, the team of theoretical physics at Universitat Rovira i Virgili in Tarragona, led by Dr. Vladimir Baulin and the experimental group of Dr. Jean-Baptiste Fleury at the Saarland University were contacted to explore this mechanism. This experimental group starts to study the conditions of trehalose delivery through the cell membrane. They designed a microfluidic experiment to form phospholipid bilayer systems, which can be considered as artificial cell membranes. With this experimental setup, they explored the interaction of individual nanoparticles with such an artificial membrane. Using a combination of optical fluorescent microscopy and electrophysiological measurements, they show that these nanoparticles did not formed any pores or damages to the artificial membrane when put in contact with it (that would explain the trehalose delivery). They show that these nanoparticles were strongly adhering to the artificial membrane, and could even measure the corresponding adhesion energy. Then Dr. Baulin investigate with computer simulations, the effect of this strongly adhesive nanoparticle on what they call a “perfect bilayer” that replaced the red blood cell membrane. Based on their calculations, the team of Dr. Baulin observed that for the adhesion energy measured experimentally, these nanoparticles are able to stretch intensively the membrane locally around the nanoparticle and thus enable a massive transfert of trehalose through the artificial membrane.
This discovery of low-cost red blood cells cryopreservation with very high survival rate allows new pathway to clinical procedures. The mechanism of trehalose delivery mediated by the membrane permeation due to the presence of nanoparticle opens interesting perspectives in biotechnology and in particular for drug delivery.
Reference: Martin Stefanic, Kevin Ward, Harvey Tawfik, Ralf Seemann, Vladimir Baulin, Yachong Guo, Jean-Baptiste Fleury, Christophe Drouet,” Apatite nanoparticles strongly improve red blood cell cryopreservation by mediating trehalose delivery via enhanced membrane permeation”, Biomaterials, Volume 140, 2017, Pages 138-149, http://dx.doi.org/10.1016/j.biomaterials.2017.06.018.
More news about: SNAL
