07/11/2024
Drug shown to be effective in preventing dialysis in children with hypercholesterolaemia
Homozygous familial hypercholesterolaemia causes children to have blood cholesterol levels that are dangerous for their health. A study led by the URV has shown that a pill, lomitapide, can reduce cholesterol in these children by half
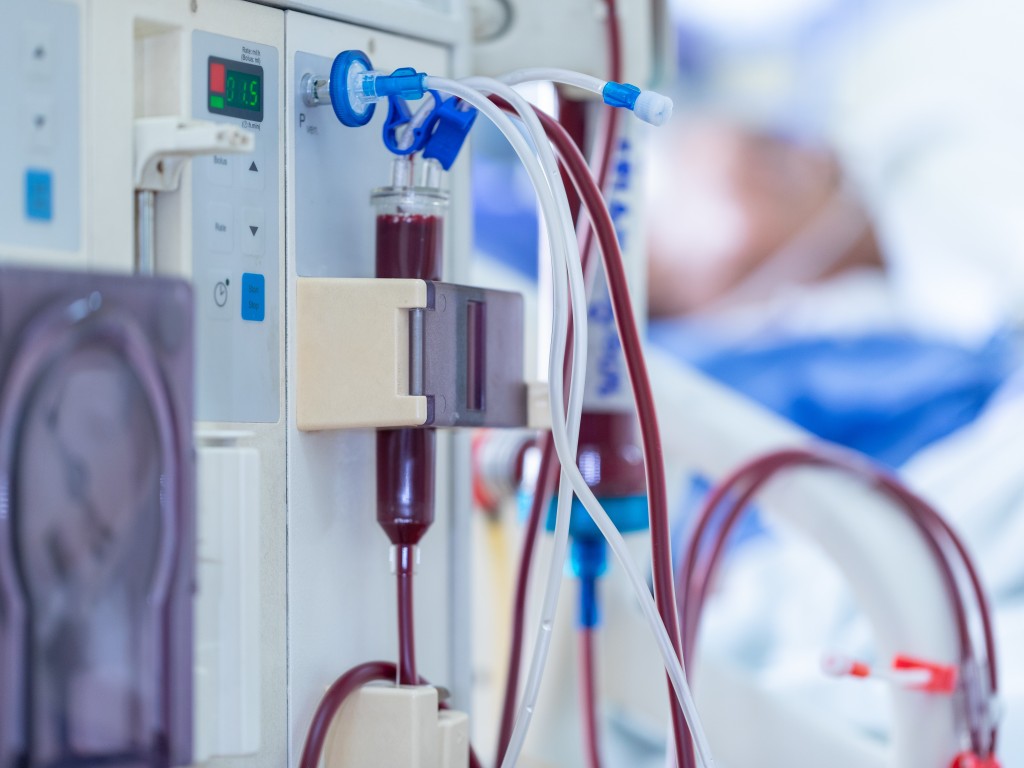
Homozygous familial hypercholesterolaemia causes children to have blood cholesterol levels that are dangerous for their health. A study led by the URV has shown that a pill, lomitapide, can reduce cholesterol in these children by half
A clinical trial led by the Vascular Medicine and Metabolism Unit of the Universitat Rovira i Virgili (URV) and Sant Joan University Hospital in Reus (HUSJR) has demonstrated the efficacy of lomitapide in reducing LDL cholesterol in children with homozygous familial hypercholesterolemia (HoFH) over a period of six months. The drug, which is approved for use in adults but not yet in children, reduced blood cholesterol by more than 50% in patients aged 5-18 years with this genetic disease. The research, which is now focusing on the longer-term safety of the drug, has involved teams for Italy, Germany, Spain, Saudi Arabia, Tunisia and Israel and should improve the quality of life of children with HoFH.
Homozygous familial hypercholesterolaemia is a rare inherited condition that affects two children per million and causes blood cholesterol concentrations above 500 mg/dl. Sufferers develop cardiovascular disease by the age of twelve on average and, if left untreated, life expectancy is approximately eighteen years. In these cases, early diagnosis is essential to avoid mortality and morbidity. Unfortunately, the usual drugs to treat cholesterol are not effective in this disease and other treatments are required which for younger patients are limited and, in some cases, not approved for paediatric use.
As a result, children with HoFH are rarely able to get their cholesterol down to healthy levels with standard treatments and end up requiring more invasive interventions such as LDL-apheresis treatment, a type of dialysis that reduces blood cholesterol. To undergo the treatment, the child has to make regular visits to a medical centre, a procedure that disrupts family routines and diminishes their quality of life.
Faced with this problem, the search for innovative therapies is crucial. One example is treatment with lomitapide, a drug that reduces the formation of a type of blood fat, a precursor of the LDL cholesterol that affects HoFH patients. The drug is administered orally and is compatible with traditional treatments, and although it has been found to significantly reduce blood cholesterol in adults when used in conjunction with a low-fat diet, until now there has been insufficient evidence of its effectiveness in children.
‘Our intention was to determine the efficacy and safety of lomitapide in paediatric patients,’ affirmed Luis Masana, a researcher at the URV’s Department of Medicine and Surgery. To do this, the research group worked in coordination with other teams from within and outside Spain to carry out a clinical study of 46 children aged between five and eighteen years. For treatment with lomitapide to be successful, apart from following a strict diet, patients need to take food supplements. ‘That’s why we have a preparation phase; we have to make sure that they tolerate the diet and supplements well before moving on to the active phase,’ explained Masana, who pointed out that, of the 46 candidates, 43 were found to be suitable for the treatment.
For 24 weeks, the patients were given one lomitapide tablet a day. By the end of this period, they had recorded a significant reduction in LDL cholesterol in the children’s blood and proved its safety. ‘The results were very positive; there was an average reduction of more than 50 percent,’ said Masana. More specifically, in children aged 5 to 10 years the reduction in LDL cholesterol recorded was 56%, whereas in patients aged 11 to 18 years, the reduction was 51%. To appreciate the importance of the results, it should be borne in mind that when HoFH patients are treated with traditional drugs, their blood cholesterol is only reduced by 10-15%.
The results of this study, published in the journal The Lancet Diabetes and Endocrinology, should help the drug to be approved for paediatric use, thus saving children with the disease from having to undergo long sessions similar to dialysis to clear cholesterol from their blood. ‘Imagine you have a five-year-old child and you are told that, for the rest of their life, your child will have to go to hospital between two and four times a month to have their blood filtered for a whole morning,’ said Masana, who is now looking forward to the day in the near future when he will be able to tell these families that one pill is enough. ‘The medical change is huge,’ he says.
After having determined the efficacy and safety of using lomitapide to treat HoFH in children over a six-month period, the second phase of the study intends to determine its long-term safety. This will be a longer, 104-week phase, during which the side effects and implications of long-term use of the drug, if any, will be assessed. ‘We are currently analysing the data from the first [safety] phase, which ended last summer,’ says Masana, who expects to publish the results in the coming months.
Reference: Luis Masana, Alberto Zambon, Claus Peter Schmitt, Christina Taylan, Joenna Driemeyer, Hofit Cohen, Paola Sabrina Buonuomo, Abdullah Alashwal, Mohammed Al-Dubayee, Naji Kholaif, José Luis Diaz-Diaz, Faouzi Maatouk, Sergio Martinez-Hervas, Brian Mangal, Sandra Löwe, Tracy Cunningham. Lomitapide for the treatment of paediatric patients with homozygous familial hypercholesterolaemia (APH-19): results from the efficacy phase of an open-label, multicentre, phase 3 study. The Lancet Diabetes & Endocrinology, 2024, ISSN 2213-8587, https://doi.org/10.1016/S2213-8587(24)00233-X
