23/02/2024
A new device uses a blood sample to identify patients with high cardiovascular risk
The URV is part of the European project PoCCardio which is developing this innovative tool for quick detection that can be used in primary health-care centres.
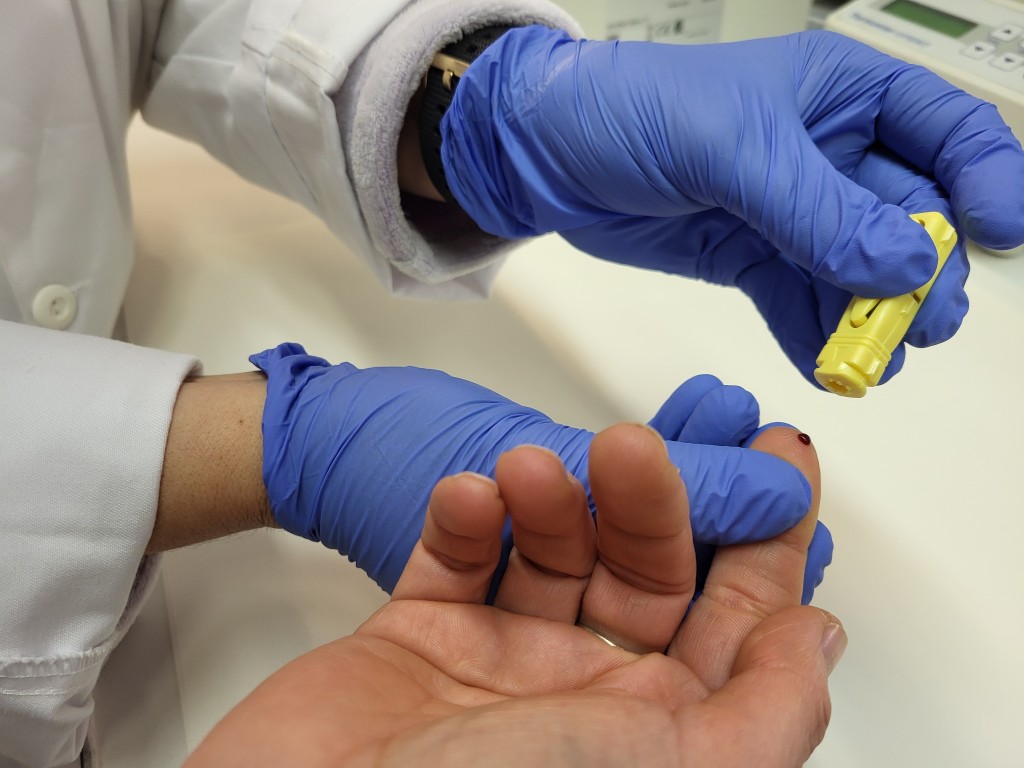
The URV is part of the European project PoCCardio which is developing this innovative tool for quick detection that can be used in primary health-care centres.
Heart attacks are one of the leading causes of morbidity and mortality around the world. And despite all the risk-assessment tools available and a variety of medicines and treatments, the prevalence of recurrent cardiovascular failure continues to be high in people with previous myocardial infarction. The Universitat Rovira i Virgili is a member of the European project PoCCardio, led by the Medical University of Graz (Austria) and six more institutions, which over the next five years aim to develop an innovative device which will make it possible to identify high-risk patients from a small sample of blood.
After a heart attack, most patients are referred to primary health-care centres for monitoring, which requires sending blood tests to external laboratories. These infrastructural constraints make it more difficult to monitor patients and prevent their cardiovascular risk from being controlled immediately. The PoCCardio project intends to design a portable and autonomous device that uses microfluidic technology to immediately analyse the blood taken from a small finger prick, as is common in diabetics. In this way, medical staff can rapidly identify the risk profile and provide the affected patients personalised care in a timely, economic, and comfortable manner.
To classify patients and make a high-quality prediction of individualised risk, the PoCCardio uses a panel of ten qualified biological markers and up to thirty-two genetic variants, measured simultaneously by a simple device that can be used at primary care points. Blood-based biomarkers are ideal for evaluating the medical condition: they are relatively simple and inexpensive, and they can play an important role in identifying risk and managing the risk factor. By using multiple disease-specific biomarkers, PoCCardio takes into account the variety of recurrent cardiovascular episodes to significantly improve prediction and adapt the treatment accordingly.
The research group Interfibio, from the URV’s Department of Chemical Engineering, is responsible for developing aptamers –single-strand DNA or RNA sequences – to detect the ten biological markers associated with cardiovascular illness. The aptamers are an inexpensive, flexible, and stable alternative to antibodies. Simultaneously, Interfibio will also develop the electrochemical platform to detect the 32 nucleotide polymorphisms associated with the illness.
Clinical validation in a multinational trial
Once the device and the biomarkers have been evaluated, a multinational study will be carried out with 35 clinical study centres in Austria, Germany, Belgium, and Poland, and over 1,800 patients who have recently had myocardial infarction. Of these, 1,500 will take part in a randomised controlled study and 300 in an observational study.
Depending on their specific biomarker pattern, patients will be assigned to a standard or intensified pharmacological treatment with approved medicines. The validation process will also include testing the device in Iran to detect differences in terms of risk factors, response to treatment and results.
The results will show if an intervention based on a risk classification driven by biomarkers and an intensified multifactorial treatment can improve the results in high-risk individuals after a heart attack, compared to current guidelines. According to Hans Peter Dimai, a researcher from the Medical University of Graz, “a complementary diagnosis made by a device such as the PoCCardio can lead to new treatment strategies for highly vulnerable patients and better results. It can also help to improve the general management of cardiovascular patients”.
The PoCCardio project is funded by the European Union through the Horizon Europe Framework Programme for Health Research and Innovation. It has a funding of 14.4 million euros and the date of completion is expected to be November 2028.
