12/05/2022
Students on the Bachelor’s Degree in Chemical Engineering construct devices self-powered with hydrogen
The students have designed plants for the production of hydrogen and on 10 June will demonstrate how their devices work and put them to the test in a competition

The students have designed plants for the production of hydrogen and on 10 June will demonstrate how their devices work and put them to the test in a competition
From which chemical reactions can hydrogen (H2) be obtained? How can volume be optimized and production still be safe? What features should a plant have to produce pure hydrogen? How do you store it for use in cars? And in self-powered moving devices?
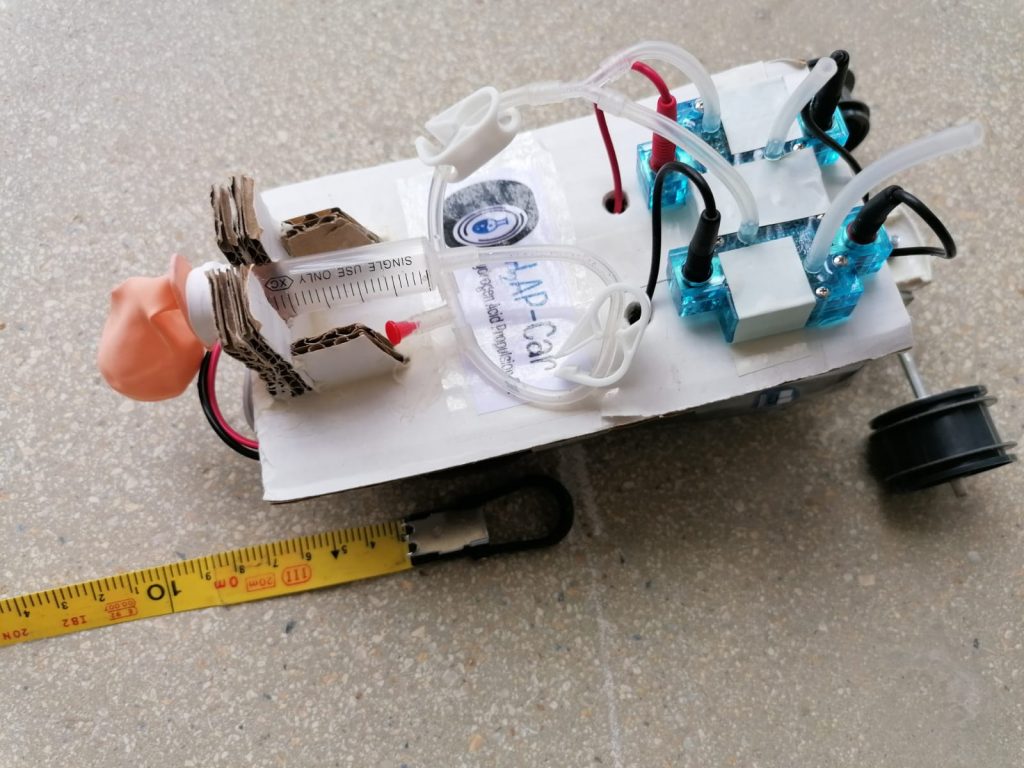
First-year undergraduates in Chemical Engineering have been working in the laboratory for weeks designing the production plant and the moving device powered only by hydrogen. On 10 June, they will have to show that they have been able to produce a car that works and that they have found a chemical reaction that will allow it to stop by itself. But the students also need to work as a team, and use a wide range of other skills: collaborative work, leadership, communication techniques, self-assessment, and conflict resolution.
It is part of the first-year integrated project, in which they put into practice in a single project everything they have learned throughout the course, including skills such as teamwork, so necessary in the world of work. For this reason, each of the ten teams has a fourth-year student who, in turn, must demonstrate that they can lead a team as part of the Team Leadership Practices course.

Ricard Garcia-Valls is the lecturer of Fundamentals of Process Engineering who thought up this practical project so that students can experiment with bibliographic research and trial and error, and use everything they have learned in the classroom. He decided to apply to the URV a type of learning that he first encountered on master’s degree courses at the University of Aalborg (Denmark), and which for several years now has been the main feature of the degrees taught by the School of Chemical Engineering: the integrated project.
The challenge for undergraduate students in Chemical Engineering this year is to move a device with hydrogen and they will be evaluated by hydrogen-producing companies. “First, they have to find the bibliography that will explain how they can produce hydrogen, first experimentally and then industrially. And then they have to design a small chemical engineering plant to produce pure hydrogen.” In the open laboratory, they test the chemical reactions, and as soon as they get their device to move, they have to optimize the volume of hydrogen so that it is produced safely, and then scale the reaction so that get enough pure hydrogen.

Some teams have opted for the most common reaction, in which the metal reacts with a concentrated acid base. But others have taken risks and tested a form of production that is still experimental: obtaining hydrogen from sunlight, which is known as photochemistry. Carlos Romera is one of the students in this team, which is assisted by researcher Alberto Puga, who is working on photocatalysis: “We were told that this issue has not been studied and we decided to try it to see how it works.” “They’ve put it all together from scratch because it’s never been done before,” Puga said. “They are showing us that at the teaching laboratory scale, H2 can be produced.”
The students have to try different steps: reactors to produce H2 from photocatalysis with UVA light of different wavelengths, a mixture of ethanol and water; a catalyst, titanium dioxide, and a cocatalyst, platinum, which reflect light and break down the molecules in alcohol to transform them into H2 and CO2. To keep the hydrogen pure, a caustic soda solution takes the CO2 from the solution and turns it into carbonate, while the H2 is transferred to another container.

All they need to do now is prove that the reaction works on the day of the contest. And they will have an added challenge: a few minutes before they run their moving device, they will be told by the jury the exact distance they have to cover. Then they will have to calibrate the car and test a second chemical reaction that will disconnect the car after the appropriate distance.
A total of about 90 students in 10 teams will have to show not only what they have learned, but also that they know how to work as a team and under pressure. Albert Escudé, a first-year Chemical Engineering undergraduate, says that when you work on the integrated project “you realize that not everything is as perfect as it is in the theoretical world, and you end up having to solve unexpected problems.”